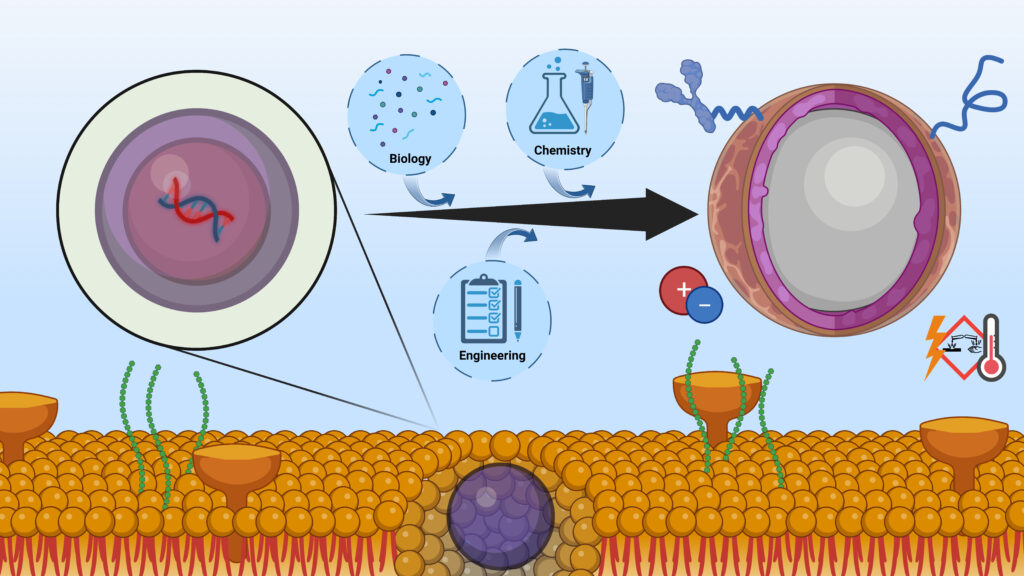
The Murthy lab is broadly interested in the fields of drug delivery and molecular imaging. The lab has an interdisciplinary environment and has expertise in organic synthesis, biochemistry and engineering. Most projects in the lab focus on the molecular design and synthesis of new delivery vectors. Current projects in the lab focus on the development of new mRNA delivery vectors and new strategies for delivering proteins and oligonucleotides into cells. We are also interested in delivering gene editing enzymes in vivo for a variety of therapeutic applications.
The Murthy lab is located in the IGI and has numerous collaborations with scientists at the IGI and at UCSF. The lab has a strong translational focus and multiple start-up companies and licenses have originated from research done in the Murthy lab.
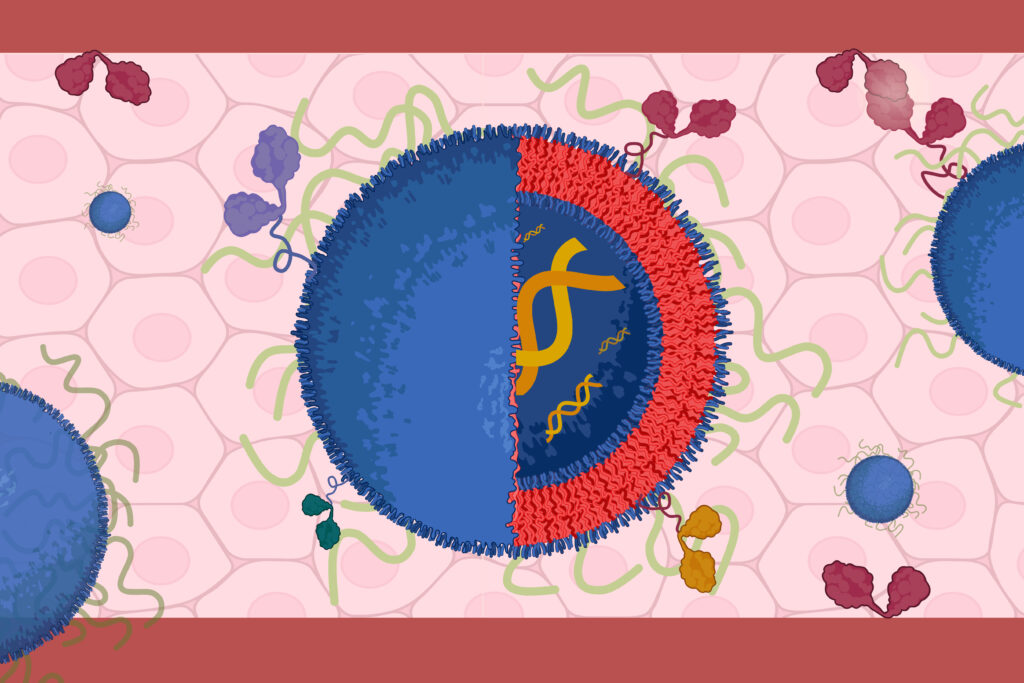
Lipid Nanoparticles Development
The Murthy laboratory has multiple projects focused on developing next generation LNPs. These projects focus on the development of LNPs that are non-immunogenic and have lower toxicity than traditional LNPs. In addition, we are also developing LNPs that disrupt endosomes efficiently and transfect non-liver organs.
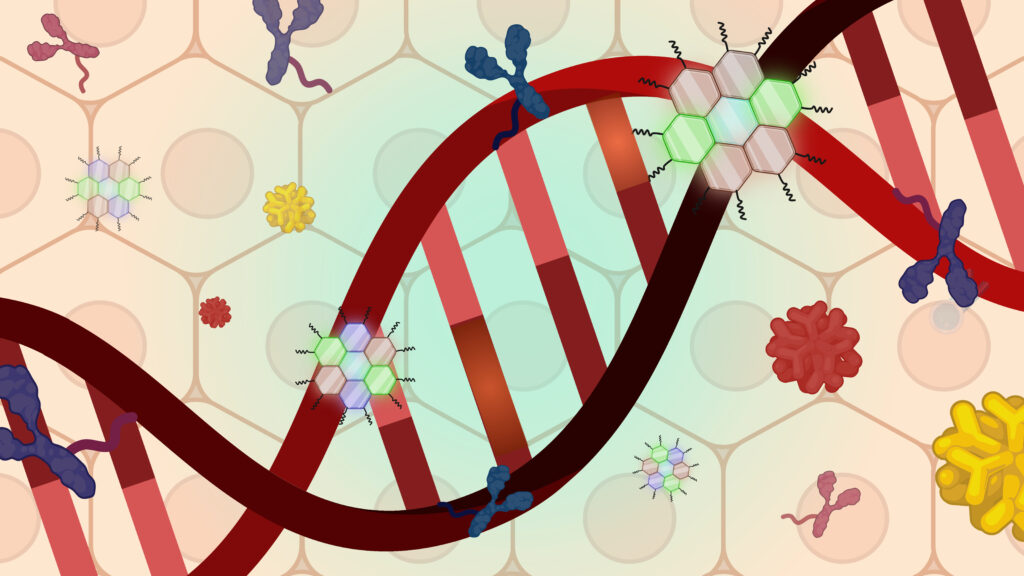
Protein/Oligo Development
The Murthy laboratory has had a long-standing interest in developing protein and oligonucleotide delivery vectors. Current projects focus on using self-immolative linkers to conjugate delivery vectors to proteins and oligos.